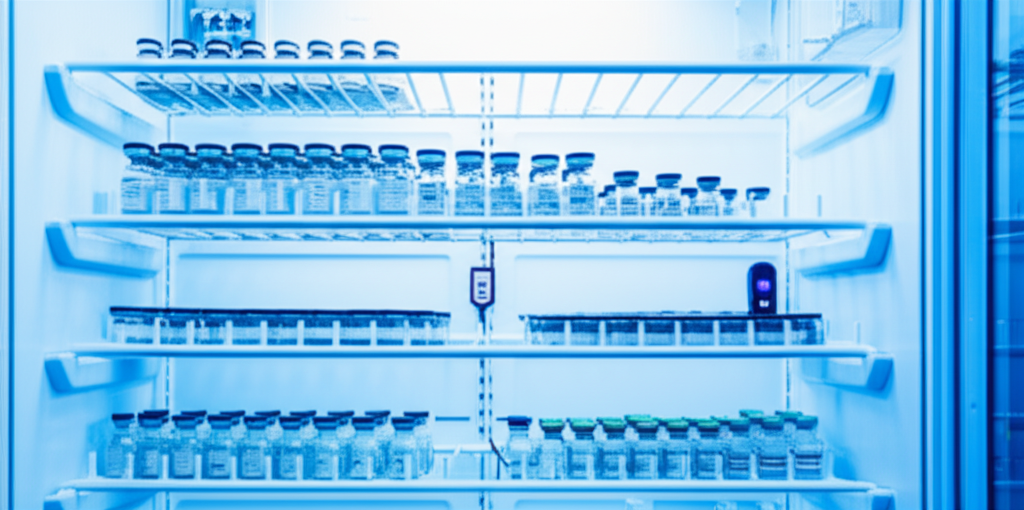
A busy afternoon at a vaccination center in Montreal when an urgent call interrupts ATEK’s technical support routine. Jonathan, the center manager, is on the line, the urgency perceptible in his voice. “The ATEK probe is reporting a temperature that’s too high, while the refrigerator displays 4 degrees. Can someone come check it?” he asks.
As an ATEK technician, I begin by reassuring Jonathan and gather additional information to identify the source of the problem.
Probe Accuracy
First, it’s important to remember that ATEK probes are extremely accurate, with a tolerance of ±0.05°C across the entire operating range of the devices. Our testing and experience have demonstrated that there was no significant drift from year to year, with a maximum observed variation of 0.02°C between validations, which is primarily attributed to the error of our laboratory instruments.
The probability that the temperature probe is inaccurate is therefore extremely low. In accordance with the Guidelines for Vaccine Management Standards and Practices, it is crucial to ensure that all devices used have a traceability certificate indicating they have been calibrated with a calibration accuracy of no more than 1°C. Probe accuracy can therefore be ruled out as the cause of the problem.
Probe Position
In discussing with Jonathan, I confirmed that the ATEK probe was already positioned correctly. ATEK’s installation team had initially placed the probe and glycol vial in the center of the refrigerator, in accordance with the recommendations of the Guidelines for Vaccine Management Standards and Practices.
This central position is strategic because it helps obtain a more representative measurement of average conditions inside the refrigerator, minimizing the effects of potentially colder or warmer zones near walls or ventilation systems. This rules out probe position as the cause of the reported temperature issue.
Product Spacing
During initial diagnosis, Jonathan confirmed that the refrigerator had recently been filled following a large vaccine delivery. This new arrangement could be causing alerts if air circulation had been obstructed.
The Guidelines for Vaccine Management Standards and Practices recommend maintaining a space of 5 to 8 cm between baskets and placing vaccines in perforated baskets, grouped by product type, to facilitate uniform cold distribution.
The ATEK probe, though already correctly positioned, had been isolated by newly stacked vaccines, which prevented air circulation and affected its measurements. Furthermore, the overloading of boxes and bags in the refrigerator had created a zone where the refrigerator’s probe, though displaying 4 degrees, did not represent uniform temperature throughout the entire refrigerator.
This created a situation where, despite correct reading at the specific location of the refrigerator’s probe, actual conditions inside could vary and endanger the integrity of stored products.
By recognizing this problem and reorganizing the space to ensure proper air circulation, the temperature measured by the ATEK probe moved closer to that of the refrigerator. This action not only resolved the temperature alerts but also ensured that storage conditions remained optimal and safe for vaccines, confirming the importance of strictly following storage and monitoring guidelines stipulated by vaccine management standards.
Conclusion
This case illustrates how crucial it is not to overload refrigerators and to strictly follow the Guidelines for Vaccine Management Standards and Practices to ensure the safety and effectiveness of stored vaccines.
![Securing your Laboratory During Extended Absences [Practical Guide]](/images/blog/securing-laboratory-absences.png)