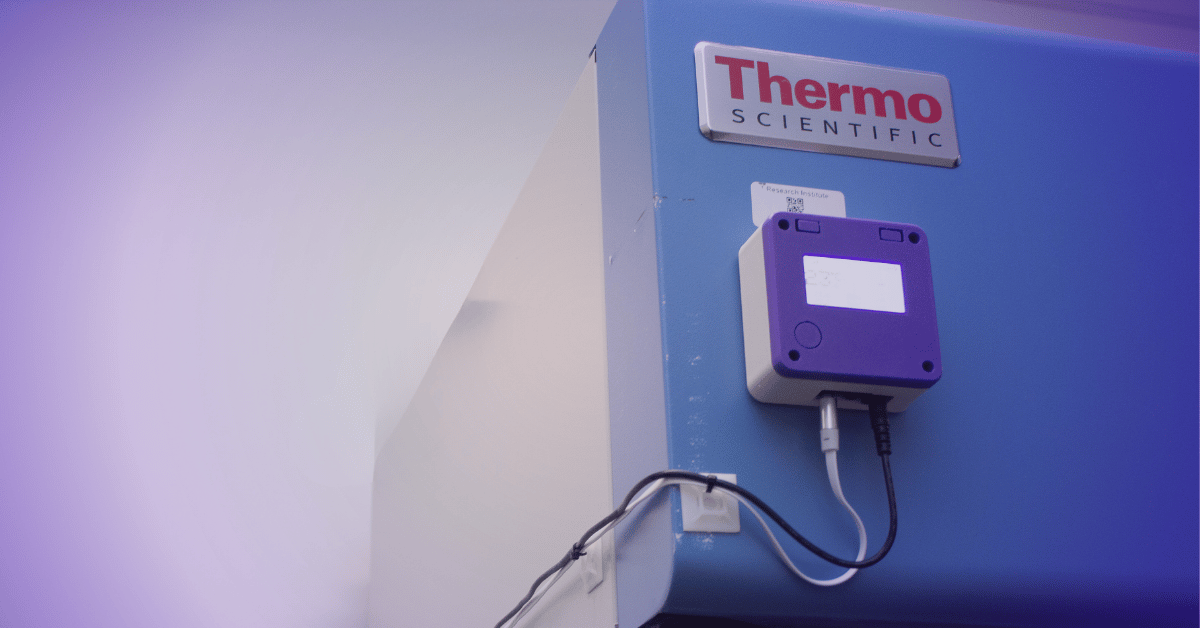
Regulated facilities-pharmacies, laboratories, hospitals, research centers-face stringent compliance mandates. Environmental monitoring of temperature, humidity, pressure, and air quality must be continuous and rigorous. Manual management creates significant risks through spreadsheet errors, missed readings, and undocumented incidents.
The Compliance Challenge
Regulatory authorities demand:
- Continuous, timestamped, secure data logging - Every measurement must be recorded with precise timestamps
- Immediate threshold breach alerts - Instant notification when conditions exceed acceptable ranges
- Clear corrective action documentation - Full traceability of all interventions
- Comprehensive, exportable audit reports - Ready-to-present documentation for inspectors
- Tamper-proof long-term data storage - Secure archiving that meets retention requirements
Manual processes dramatically increase human error risk, jeopardizing product integrity and organizational standing.
ATEK’s Automated Solutions
24/7 Continuous Monitoring
High-precision, certified sensors automatically record parameters with timestamps and secure storage. No more manual readings or paper logs-every data point is captured automatically.
Real-Time Alerts
Instant notifications via SMS, email, or push notifications enable immediate intervention during off-hours. When a freezer starts failing at 3 AM, you’ll know about it within seconds.
Automatic Documentation
Field teams log actions directly within the platform with supporting evidence and complete traceability. Every corrective action is documented with timestamps, user identification, and outcome details.
Simplified Audits
Auto-generated reports with historical data, graphs, and digital signatures streamline regulatory inspections and certification renewal processes. What used to take days of preparation now takes minutes.
Secure Archiving
Encrypted cloud storage with redundancy protects records and meets retention requirements. Your data is safe, accessible, and compliant with data integrity regulations.
Additional Support
ATEK provides:
- Calibrated sensors with NIST-traceable certificates
- User-friendly interfaces designed for real-world workflows
- Responsive technical support available 24/7
- Customized configurations for your specific needs
- Staff training aligned with regulatory frameworks (GxP, FDA 21 CFR Part 11, ISO 17025)
Key Benefits
- Substantially reduced non-compliance risk - Automated systems eliminate human error
- Constant audit readiness - Always prepared for inspections
- Minimized administrative workload - Focus on your core mission
- Proactive product protection - Catch issues before they become problems
- Stress-free continuous compliance - Peace of mind for your entire team
Explore specific regulatory requirements in our compliance guides for FDA 21 CFR Part 11, ISO 17025, and GAMP 5. See ATEK’s validation services for IQ/OQ/PQ protocols.
Ready to simplify your regulatory compliance? Contact our team to learn how ATEK can transform your environmental monitoring.